介孔碳化钨 /炭纳米复合材料的制备与表征(英文)
1.中南林业科技大学材料科学与工程学院,湖南长沙 410004; 2.竹业湖南省工程研究中心,湖南长沙 410004
Synthesis and characterization of mesoporous tungsten carbide/carbon nanocomposites
1. College of Materials Science and Engineering, Central South University of Forestry & Technology, Changsha 410004, Hunan, China; 2. Hunan Provincial Engineering Research Center of Bamboo Industry, Changsha 410004, Hunan, China
基金项目:国家林业公益性行业科研重大专项 (201204704);国家自然科学基金项目 (30871976,31070496);国家 “十二五 ”科技计划课题 (2012BAD24B03);教育部博士点基金项目 (20114321110005);湖南省科技重大专项 (2011FJ1006);湖南省杰出青年基金项目 (09JJ1003);人力资源和社会保障部留学归国人员科技活动择优资助项目;中南林业科技大学引进人才科研项目 (104-0217);中南林业科技大学木材科学与技术国家重点学科资助项目
作者简介:夏燎原 (1977—),男,湖南邵阳人,讲师,博士,现从事多孔材料和阻燃材料方面研究通讯作者:胡云楚 (1960—),男,湖南湘潭人,教授,博士,博士生导师,主要从事材料化学和阻燃材料方面的研究吴义强 (1967—),男,河南固始人,教授,博士,博士生导师,主要从事木材材性、木材功能性改良、生物质复合材料方面的研究; E-mail:wuyq0506@126.com
-
碳化钨作为一种潜在的催化剂可广泛应用于电化学催化和有机合成反应 .本文通过一种简单可行的 “软模板 ”法制备了介孔碳化钨 /炭纳米复合材料,主要包括 “油包水 ”微乳液形成、模板诱导自组装、高温碳化还原过程 .采用 X-射线衍射、透射电镜和比表面积和孔径分布等方法对材料进行了表征与分析。结果 表明,该复合材料具有蠕虫状的介孔结构、高的比表面积、碳化钨粒子 (约 40 nm)均匀的分布在炭载体上 .介孔碳化钨 /炭纳米复合材料可用于燃料电池、化学传感器和电催化有机合成反应 .
Tungsten carbide (WC) can be used as potential catalysts for various electrocatalyst and chemical reactions. A simply soft-template route to fabricate mesoporous tungsten carbide/carbon (WC/C) composites was prepared by W/O emulsion and triblock copolymer self-assembly strategies, followed by a high-temperature carbothermal reduction. XRD, TEM and BET surface area and pore size distribution techniques were employed to characterize the mesoporous WC/C nanocomposites. The results show that the resultant materials have wormlike mesostructure, nnaoscale (about 40 nm) and well-dispersed tungsten carbide particles, and high surface areas. Furthermore, the mesoporous WC/C nanocomposites could have great potential applications in fuel cell electrocatalyst, sensors and organic synthesis reactions.
Introduction
Tungsten carbide (WC), as a special type ofcarbide materials, can be used as potential catalysts forvarious chemical and electrochemical reactions, such asmethanol oxidation[1-3], oxygen reduction[4-5], nitrophenol oxidation[6-7], and hydrogen evolution[8-9]. Theseimportant applications are attributed to its platinum-likecatalytic behavior, stable in acidic and basic solutions,relatively low cost and high resistance to poisoning[1, 10-15]. However, some critical problems are limited to develop highly efficient WC catalyst. On the one hand, the WC alone shows much lower catalytic activity than that of noble metal and unstable in some environments. On the other hand, the WC prepared by direct carburization of tungsten species at high-temperature usually has low surface area, thereby restricting its application as catalysts in the related fields.
To overcome above-mentioned drawbacks, an efficient solution is to form mesoprous tungsten carbide/carbon (WC/C) nanocomposites. As we know, the catalytic performances of a catalyst are strongly dependent on the dispersion and accessibility of the active sites. Therefore, nano-structured carbon[16-18], active carbons[4,19], especially mesoporous carbon[20-21] have been widely used as catalyst supports to improve the catalytic activity and durability of the WC, which is attribute to their particular structures and high surface area. Up to now, much effort has been focused on preparation of the mesoprous WC/C nanocomposites using various methods, such as impregnating method[4,20], hydrothermal reaction[3,17,22] and templatereplicating[21,23], in which carbon reacts with tungsten or tungsten oxides under reductive environments, is the most universal approach. Fore instance, Raman et al. [3] and Wang et al. [17] synthesized mesoporous WC phase by a surfactant-assisted hydrothermal reaction route. However, the resultant materials showed relatively small pore diameters and low surface areas as compared to common mesoporous materials by similar surfactant routes. Commonly, the hard-template method is also regarded as an effective way to prepare ordered mesoporous WC/C composites[16,21,23]. Nevertheless, the synthesis procedures are rather multi-steps, high-cost, time-consuming, and a collapse of the mesostructure regularity would usually occur during the removal of the hard templates. Although excellent progress has been made in the preparation of the WC/C composites, as far as we know there is no report up to now regarding the synthesis of mesoporous WC/C composites by a soft-template method, because it is difficult to find proper precursors for the self-assembly of precursors and surfactants through sol-gel process. Thus, it is still a great challenge to provide a facile soft-template route for a mass of fabrication of the mesoporous WC/C nanocomposites with well-defined mesostructures and well-dispersed behavior for the practical applications. In this work, for the first time, we demonstrate a simple soft-template method to prepare the mesoporous WC/C nanocomposites with wormlike mesostructures, small-sized (about 40 nm) and finely dispersed tungsten carbide particles, and high surface areas. The materials have great potential for applications in fuel cell electrocatalyst, sensors and organic synthesis reactions.
1 Experimental
1.1 Materials
All chemicals were used as received without further purification. Triblock copolymer Pluronic F127 (EO106PO70EO106, Mw = 12 600) and ammonium tungsten oxides hydrate (ATOH) were bought from Aldrich. Phenol, formalin solution (37%), melamine, hydrogen chloride (HCl, 37%), sodium hydroxide and ethanol were obtained from Guangzhou chemical company.
1.2 Synthesis of mesoporous WC/C nanocomposites
In a typically synthesis, 5.0 g PFM resin precursors were dissolved in 20.0 g ethanol and 10.0 g H2O solution at 35 ℃ . Then, 2.0 g of F127 was added and stirred for 30 min. A certain amount (0.5~ 2.0 g) of ATOH aqueous solution (33.3 wt. %) was dropped into the above mixture under vigorous stirring for 30 min. Then, the mixture was cast onto glass dishes, followed by evaporation of mixture solution for 24 h at 35 ℃ . The resulting sticky films were further thermosetted at 105 ℃ for 24 h. Last, the brown colored films were placed in an alumina boat and calcined at 900℃ for 3 h under (N2+H2) atmosphere in a tubular furnace.
1.3 Characterization
X-ray diffraction (XRD) patterns were recorded by a Rigaku 2 200 diffractometer using Cu Kα radiation source (40 kV, 30 mA). Transmission electron microscopy (TEM) observation was conducted on a JEM-2010HR machine operating at200 kV. Nitrogen adsorption-desorption isotherms were measured at 77 K on a Micrometrics ASAP 2010 apparatus.
2 Results and discussion
Table1 summarizes the experimental conditions and structural parameters of all the mesoporous WC/C nanocomposites. The effects of the tungsten source were investigated by varying the amount of ATOH while fixed the carburizing agent contents. Accordingly, the weight ratios of H2O/EtOH were altered to ensure the ATOH finely dispersed in mixture solutions.
Low-angle X-ray diffraction (XRD) patterns of the mesoporous WC/C nanocomposites are displayed in Figure 1. Only a broad peak at low angle is perceivable, suggesting that products have a disordered wormlike framework[24]. Moreover, with the increasing the amount of ATOH, the diffraction peaks become gradually broader and even disappear. This indicates that the mesostructure becomes inferior, probably resulting from the precursors-micelles assembly slightly disturbed at higher ATOH content.
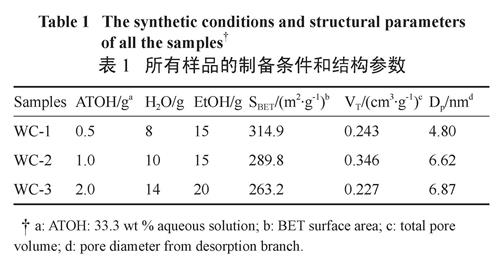
表1 所有样品的制备条件和结构参数
Table 1 The synthetic conditions and structural parameters of all the samplesThe typical wide-angle X-ray diffraction (WXRD) patterns of the mesoporous WC/C nanocomposites are shown in Figure 2. It can be clearly seen that the WXRD patterns display three distinct diffraction peaks at 31.49, 35.68 and 48.36,which can be index as the (001), (100), (101) planes of hexagonal WC phase (PDF#51-0939). In addition, several resolved diffraction peaks present at 2θ =34.44, 38.06, 39.44 and 52.23, which can be assigned to 100, 002, 101, 102 reflections attributed to W2C phase (PDF #20-1315). These results indicated that the products are mainly composed of WC and W2C phase together with small amounts of WC1-x, which is depending on the amount of ATOH.
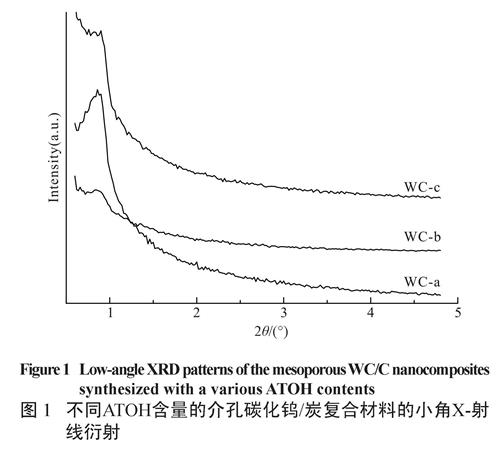
图1 不同ATOH含量的介孔碳化钨/炭复合材料的小角X-射线衍射
Fig.1 Low-angle XRD patterns of the mesopoorus WC/C nanocomposites synthesized with a various ATOH contents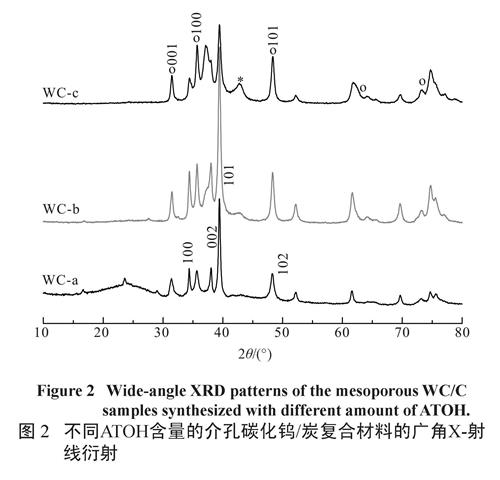
图2 不同ATOH含量的介孔碳化钨/炭复合材料的广角X-射线衍射
Fig.2 Wide-angle XRD patterns of the mesoporous WC/C samples synthesized with different amount of ATOH.As shown in Figure 3A, the tungsten carbide particles (dark spots) are well-dispersed into the carbon supporter, and most of the particles are about 40 nm (Figure 3B). Furthermore, the high-resolution transmission electron microscope (HRTEM) image shows that the WC/C nanocomposites have wormlike structures (Figure 3 C), in good agreement with the analysis results of SXRD measurements. Evidently, this route provides a feasible way to synthesize mesoporous WC/C nanocomposites with desire mesostructure and well-dispersed tungsten carbide particles. To further clarify the textural properties of the wormlike mesoporous WC/C nanocomposites, nitrogen sorption experiment were measured at 77 K. Figure 4 shows the N2 adsorption-desorption isotherms of all the mesoporous WC/ C nanocomposites. These samples exhibit a typical type-IV isotherm with an obvious H3 hysteresis loop, indicating the existence of disordered mesostructure, which is consistent with above the XRD analysis and TEM observation (see Figure 3).
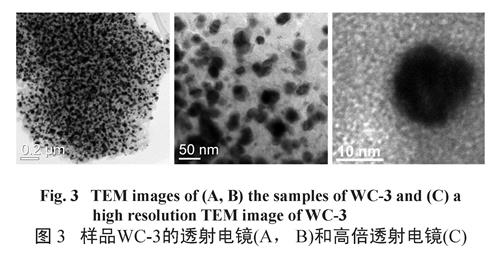
图3 样品 WC-3的透射电镜 (A, B)和高倍透射电镜 (C)
Fig.3 TEM images of (A, B) the samples of WC-3 and (C) a high resolution TEM image of WC-3According to calculation results obtained by BJH method, the pore size distribution (PSD) curves of the mesoporous WC/C nanocomposites display a relatively narrow peak at 3.9 nm and broad peak at 9.2 nm (see Figure 4, inset), which can be ascribed to the wormlike mesoporous and the large interstitial nanopores observed in the TEM images (see Figure 3 B and A, marked as arrow), respectively. As shown in Table 1, the BET surface areas of the mesoporous WC/ C nanocomposites gradually decreases from 314.9 to 263.2 m2/g with increasing the ATOH from 0.5 to 2.0. This decrease is mainly attributed to density increases with rising tungsten (W) content.
3 Conclusion
(1) In present work, we have developed a simply soft-template route to fabricate mesoporous WC/ C nanocomposites by W/O emulsion and triblockcopolymer self-assembly strategies, followed by high temperature carbothermal reduction. In the synthesis process, PFM resin and ATOH are employed as the precursors, Pluronic F127 as a template and H2O/ ethanol as the solvent, respectively.
(2) The resultant materials possess a wormlike mesosturcture, high surface areas and nano-scale tungsten carbide particles finely dispersed into carbon support. In addition, the wormlike mesoporous WC/ C nanocomposites are expected to have potential applications in electrochemical, sensors and organic synthesis reactions.
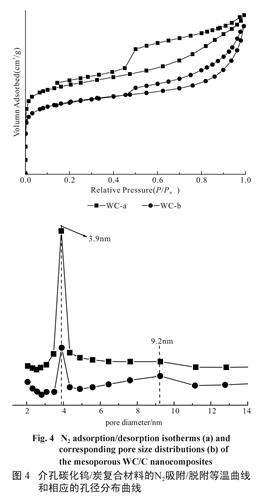
图4 介孔碳化钨/炭复合材料的N2吸附/脱附等温曲线和相应的孔径分布曲线
Fig.4 N2 adsorption/desorption isotherms (a) and corresponding pore size distributions (b) of the mesoporous WC/C nanocomposites-
参考文献
- [1] Ganesan R, Lee J S. Tungsten Carbide Microspheres as a NobleMetal-Economic Electrocatalyst for Methanol Oxidation[J]. Angew. Chem. Int. Ed., 2005, 44: 6557.
- [2] Mclntyre D R, Burstein G T, Vossen A. Effect of carbon monoxide on the electrooxidation of hydrogen by tungsten carbide[J].Journal of Power Sources, 2002, 107: 67-73.
- [3] Ganesan R, Ham D J, Lee J S. Platinized mesoporous tungsten carbide for electrochemical methanol oxidation[J]. Electrochem. Commun., 2007, 9: 76-79.
- [4] Meng H, Shen P K. The beneficial effect of the addition of tungsten carbides to Pt catalysts on the oxygen electroreduction [J]. Chem. Commun., 2005, 31:4408.
- [5] Meng H, Shen P K. Tungsten Carbide Nanocrystal Promoted Pt/ C Electrocatalysts for Oxygen Reduction[J]. Journal of Phys. Chem. B, 2005, 109: 2705.
- [6] Li GH, Ma C A, Tang J Y, et al. Preparation and electrocatalytic property of WC/carbon nanotube composite[J]. Electrochim. Acta, 2007, 52: 2018-2023.
- [7] Li GH, Ma C A, Zheng Y F, et al. Preparation and electrocatalytic activity of hollow global tungsten carbide with mesoporosity[J]. Micropor. Mesopor. Mater., 2005, 85: 234-240.
- [8] Schlapbach L, Zuttel A. Review article Hydrogen-storage materials for mobile applications[J]. Nature, 2001, 414: 353-358.
- [9] Wu M, Shen P K, Wei Z D, et al.High activity PtPd-WC/C electrocatalyst for hydrogen evolution reaction[J].Journal of Power Sources, 2007, 166: 310-316.
- [10] Levy R L, Boudart M. Genetic Control of Susceptibility to Experimental Allergic Encephalomyelitis in Rats[J].Science, 1973, 181: 873.
- [11] Bodoardo S, Maja M, Penazzi N, et al. Oxidation of hydrogen on WC at low temperature[J].Electrochim. Acta, 1997, 42: 2603-2609.
- [12] Barnett C J, Burstein G T, Kucernak A R J, et al. Electrocatalytic activity of some carburised nickel, tungsten and molybdenum compounds[J]. Electrochim. Acta, 1997, 42: 2381-2388.
- [13] Chhina H, Campbell S, Kesler O. Thermal and electrochemical stability of tungsten carbide catalyst supports[J]. Journal of Power Sources, 2007, 164: 431-440.
- [14] Christian J B, Smith S P, Whittingham M S, et al.Tungsten based electrocatalyst for fuel cell applications[J].Electrochem. Commun., 2007, 9: 2128-2132.
- [15] Jeon M K, Daimon H, Lee K R, et al. CO tolerant Pt/WC methanol electro-oxidation catalyst[J].Electrochem. Commun., 2007, 9: 2692-2695.
- [16] Bosco J P, Sasaki K, Sadakane M, et al. Synthesis and Characterization of Three-Dimensionally Ordered Macroporous (3DOM) Tungsten Carbide: Application to Direct Methanol Fuel Cells[J]. Chem. Mater., 2010, 22: 966-973.
- [17] Wang Y, Song S Q, Shen P K, et al.Nanochain-structured mesoporous tungsten carbide and its superior electrocatalysis[J]. Journal of Mater. Chem., 2009, 19: 6149-6153.
- [18] Zhou X S, Qiu YJ, Yu J, et al.Tungsten carbide nanofibers prepared by electrospinning with high electrocatalytic activity for oxygen reduction[J]. Int. Journal of Hydrogen Energ., 2011.In Press.
- [19] Ji N, Zhang T, Zheng M Y, et al. Catalytic conversion of cellulose into ethylene glycol over supported carbide catalysts[J]. Catal. Today, 2009,147: 77-85.
- [20] Zhu Q, Zhou S H, Wang X Q, et al.Controlled synthesis of mesoporous carbon modified by tungsten carbides as an improved electrocatalyst support for the oxygen reduction reaction[J]. Journal of Power Sources, 2009, 193: 495-500.
- [21] Zhang Y H, Wang A Q, Zhang T. A new 3D mesoporous carbon replicated from commercial silica as a catalyst support for direct conversion of cellulose into ethylene glycol[J]. Chem. Commun., 2010, 46: 862-864.
- [1] Ganesan R, Lee J S. Tungsten Carbide Microspheres as a NobleMetal-Economic Electrocatalyst for Methanol Oxidation[J]. Angew. Chem. Int. Ed., 2005, 44: 6557.
中南林业科技大学学报
